

The University of Limerick’s Department of Chemical Sciences is pleased to announce the launch of its newly installed, large-scale, interactive periodic table display – the first of its kind in any third level institution in Ireland.
The display measures 2.4 metres wide by 2 metres tall and contains an impressive array of pure element samples (solids, gases and liquids) plus related minerals, ores, artefacts and everyday items, all housed securely within individually lit, encased, 120mm-sided cubes. It also contains five glass tubes blown in the shape of the chemical symbols of the first five noble gases and filled with the corresponding gas to demonstrate their distinctive colour and intensity when illuminated electrically. An inbuilt interactive touchscreen displays many interesting facts about each element, as well as descriptions of the contents of each element’s cube and videos of experiments involving the elements.
The display was custom-designed, fabricated and installed with great care and attention to detail by a specialist company based in Madrid, Spain. A fundraiser helped to cover the cost of the display whereby people donated between €50 and €400 to ‘adopt’ an element and have a short personalised inscription laser-engraved on the inside wall of their adopted element’s cube. In this way, many donors took the opportunity to use their inscriptions to acknowledge family members, classmates, friends, work colleagues or mentors, or to remember those who have passed on. A variety of companies, organisations and institutions also came on board as corporate sponsors, and their logos are presented prominently on the display’s two side panels.
The display will serve as a permanent, high-impact attraction to better promote science among UL’s undergraduate students and among secondary and primary school-goers in the Mid-West region and beyond. To request a viewing, please fill out the form below.
The Periodic Table of the Elements is an organised way of arranging and presenting the different types of fundamental substances (known as ‘elements’) that are used to make absolutely everything in our Universe. For example, common table salt is made from units (namely ‘atoms’) of the elements sodium and chlorine; sugar is made from atoms of the elements carbon, hydrogen and oxygen. Water for our coffee is made from atoms of the elements hydrogen and oxygen, and the stainless steel spoon used to stir the coffee is made from atoms of the elements iron, carbon, chromium, nickel and others. Copper pipes are made from, yes, you’ve guessed it, atoms of the element copper.
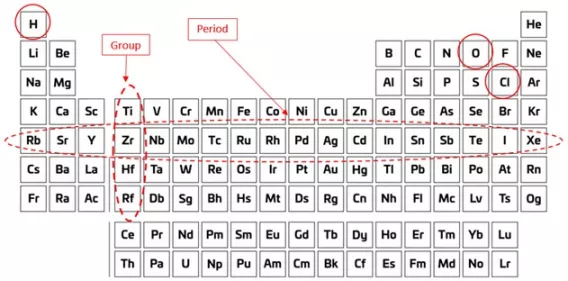
Students usually encounter the periodic table during science class as a two-dimensional grid of squares printed on a page, where each element’s unique chemical symbol is displayed inside its designated square (‘O’ or oxygen, ‘H’ for hydrogen, ‘Cl’ for chlorine, and so on). Importantly, each element is assigned to a specific square on the grid (or ‘table’) depending on its ‘atomic number’; this is the number of protons in the centre (or ‘nucleus’) of its atoms. For example, every atom of oxygen in the Universe has eight protons in its nucleus, therefore oxygen occupies the eighth square on the table (when starting at the top-left square and moving across from left to right). Broadly speaking, arranging the elements in increasing order of atomic number means that you will find the smaller, lighter elements in the squares towards the top of the table, and the larger, heavier elements in the squares towards the bottom. Of equal importance, though, is that the vertical stacks of squares (known as ‘groups’) and the horizontal rows of squares (known as ‘periods’) have been arranged deliberately so that the elements within them exhibit certain trends or patterns in their chemical and physical behaviour. For example, elements in Group 1 (the leftmost group on the table) react with water to produce flammable hydrogen gas. This reaction proceeds mildly enough for element lithium (Li) near the top of the Group 1 but gets progressively livelier for the elements all the way down the group to caesium (Cs), which reacts explosively.
The Russian scientist Dmitri Mendeleev used such recurring (or ‘periodic’) patterns in elements’ behaviour down the groups and across the periods to draw up the first periodic table of the then-known elements in 1869. Since then, many more elements have been discovered and duly assigned to their allocated squares on the table based on their atomic numbers. Today, the Periodic Table of the Elements presents the ordered arrangement of some 118 elements. Of these, around 90 are naturally occurring and the remainder have been made in laboratories.
One of the things that makes the periodic table so powerful is that it brings a level of insight to understanding and predicting a given element’s chemical and physical behaviour depending on its position on the table. This has proved invaluable to countless scientists the world over; and it will continue to do so for as long as scientists push the boundaries of scientific discovery.