What are MOMs?
MOMs, also known as porous coordination polymers (PCPs)3 or metal-organic frameworks (MOFs),4 are open networks generally comprised of metal cations, metal cluster molecular building blocks (MBBs)5 or metal-organic polyhedral supermolecular building blocks (SBBs)2,6 connected by organic or inorganic linkers. They are a class of porous materials that have captured the imagination of researchers worldwide because they can be designed from first principles and they exhibit unprecedented surface area.
The amenability of MOMs to design comes from using MBBs or SBBs that serve the geometric role of the node in a network and the geometry of the node is propagated by linkers. The design of MOMs therefore begins by judiciously selecting the right combination of metal/MBB/SBB and linkers to afford control of the symmetry and dimensions of the resulting network structure.
Why are MOMs of interest?
The use of metal cluster MBBs to afford framework solids with unprecedented permanent porosity,7 as exemplified by prototypal materials such as HKUST-1 (See Figure)8 and MOF-5,9 has captured the imagination of materials scientists worldwide. This is because when such permanent porosity is coupled with the inherent modularity of MOMs, they become relevant to several contemporary challenges related to the environment and energy sustainability:
- Reducing the cost of energy consumption associated with commodity production (e.g. through improved heterogeneous catalytic processes or through physisorption that will enable improved separation methods);
- Enabling more efficient, less-polluting means of energy production (e.g. carbon capture) and transportation (e.g. methane or hydrogen storage for vehicular transport);
- Development of electrically conductive porous materials (e.g. for use in heat pumps, photocatalysis, environmental sensing and solar energy);
- Further, such a high degree of control over structure allows for systematic study of structure/function so that iterative design processes become feasible.
More Information on MOMs
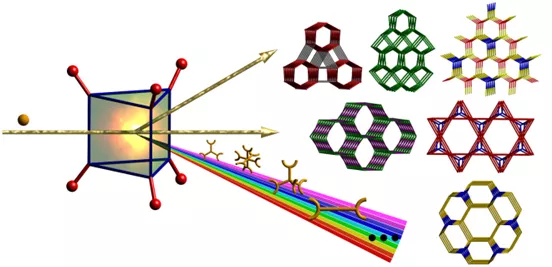
What is 2-Step Crystal Engineering?
In contrast to conventional one-pot synthesis, which is commonly used to synthesize metal-organic materials, MOMs, we have developed a 2-step strategy. Step 1 involves the synthesis and isolation of a decorated molecular building block (MBB). The decorated MBB contains functional groups (e.g. pyridyl or amine moieties) that are suitable for coordination to a different metal ion in step 2, thereby allowing the generation of multi-nodal MOMs that would otherwise be difficult to isolate. The first examples of such MOMs were reported in 20111 and the success of 2-step crystal engineering has since been more broadly demonstrated.2-5
Which nets can be prepared?
The first examples of multi-nodal MOMs were based upon [Cr3(µ3-O)(RCO2)]6 decorated MBBs (step 1) and by reacting with appropriate metal centers and linkers it was possible to generate a structurally diverse range of novel binodal MOMs including those based upon snx, snw, stp, acs and rtl topology.6 Further studies will address other classes of decorated MBBs as well as the generation of multi-nodal MOMs by using additional di- and tri-carboxylate ligands.
Why is 2-Step Crystal Engineering of interest?
- Charged networks that possess large solvent-accessible channels for decoration of nanopores through ion exchange are readily accessible if charged MBBs are utilized as nodes.
- The inherently modular strategy enables precise control over the assembly process and thus facilitates the generation of multi-nodal families of frameworks or platforms.
- Multi-nodal platforms are highly amenable to fine-tuning of both the nodes and the linkers, thereby facilitating systematic structure/property studies.
- 4 new platforms have been isolated thus far: asc (2), lon-e (3), stp (1,4,5) and fsb (5) networks.
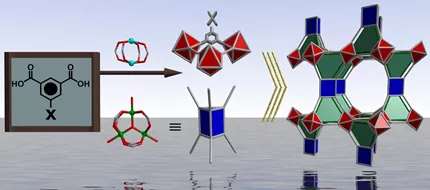
What is a lon-e net?
It is a new and versatile class of metal-organic materials (MOMs) that is based upon the augmented lonsdaleite-e or lon-e-a topology. The first examples of lon-e nets were reported in 2013.1
How are lon-e nets prepared?
Lonsdaleite-e nets are prepared according to our previously reported 2-step strategy: Step 1 involves the synthesis and isolation of a decorated molecular building block (MBB), in this case [Cr3(µ3-O)(RCO2)]6 MBBs decorated by six pyridyl moieties. The decorated MBB is suitable for coordination to a different metal cluster in step 2 (a [Zn2(CO2R)4] paddlewheel MBB), thereby allowing the generation of a family of multi-nodal MOMs.
Lonsdaleite-e nets can be thought of as being built by pillaring hexagonal 2-dimensional kagomé (kag) lattices that are in turn constructed from well-known [Zn2(CO2R)4] paddlewheel molecular building blocks (MBBs) connected by 1,3-benzenedicarboxylate (bdc) linkers.
Why are lon-e nets of interest?
- These are the first examples of axial-to-axial pillared undulating kag layers.
- The MBBs are inexpensive and readily fine-tuned; in particular, bdc moieties can be varied at their 5-position without changing the overall structure.
- Nanoscale hexagonal channels are exhibited because the kag lattices are necessarily eclipsed. lon-e nets can exhibit surface area of >1000 m2/g.
- Modularity offers systematic study of the effect of pore size and pore chemistry upon gas adsorption. Strong affinity for methane sorption was observed for bdc linkers substituted with t-butyl groups.
What is an mmo net?
It is a versatile metal organic material (MOM) platform of general formula [M(L)2(M’O4)] (M = first row transition metal; L = dipyridyl-type linker; M’O4 = angular inorganic oxyanion pillar). They can be described as self-catenated neutral nets built from [M(L)2] square grids linked by angular pillars such as CrO4-2, MoO4-2 or WO4-2. They are the first examples of 6-connected 48.67 topology nets, have been assigned RCSR code mmo after their discoverer, Mona Mohamed, and were first reported in 2012.1
How are mmo nets prepared?
mmo nets are prepared by a facile 1-step self-assembly reaction that is conducted at room temperature.
Why are mmo nets of interest?
- They are inexpensive and particularly facile to synthesize.
- Their modular nature means they can be fine-tuned at 3 sites (M, linker and M’) without changing the overall structure. This allows for systematic study of structure-property relationships.
- The prototypal mmo nets are extraordinary stable as they retain their crystallinity in water over a wide range of pH and they are stable in air and boiling water for months.
- They represent the first examples of porous MOMs based on CrO4-2, MoO4-2 or WO4-2 moieties.1-3
- The protypal mmo nets, MOFOUR-1-Ni, CROFOUR-1-Ni and WOFOUR-1-Ni, are CO2 adsorbents that outperform many other MOMs, even those with unsaturated metal centers, UMCs, or amine functionalized MOMs. This performance has been ascribed to strong quadrupole-quadrupole interactions between CO2 and the metal oxide binding sites that line the pore walls.

What is a fsb?
It is a new and versatile class of metal-organic materials (MOMs) that is based upon the four-six connected net b (fsb) topology. The first examples of fsb nets were synthesized in 2013.1
How are fsb nets prepared?
fsb nets are prepared according to our previously reported 2-step strategy: Step 1 involves the synthesis and isolation of a decorated molecular building block (MBB). The decorated MBB contains functional groups that are suitable for coordination to a different metal cluster in step 2 (here a [Zn(CO2R)2(py)2] tetrahedral MBB), together with a linear (or slightly bent) dicarboxylate linker, thereby allowing the generation of multi-nodal MOMs. fsb nets are built by connection of [Zn6(dicarboxylate)6] Supermolecular Building Blocks (SBBs) to the pyridyl moiety of the [Cr3(µ3-O)(RCO2)]6 MBBs.
Why are fsb nets of interest?
- They represent a new and versatile class of metal-organic materials.
- They are constructed from simple and inexpensive building blocks.
- They afford very high free void volume.
- Their underlying topology precludes interpenetration (not self-dual).
- fsb nets are anticipated to yield ultra-high surface areas.
- The availability of many functionalized 1,4-bdc derivatives allows for systematic fine-tuning of properties.
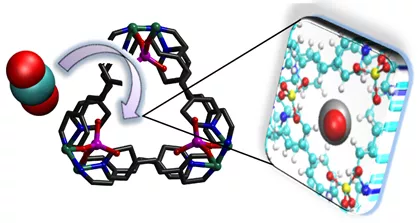
What are Porph @ MOMs?
Porphyrins are functional organic compounds that play an important role in biochemistry and have also found industrial applications as dyes and catalysts. Porph @ MOMs exemplify a class of metal-organic materials (MOMs) in which porphyrin molecules or ions are encapsulated by cages that are within the MOM structure. The porphyrin is thereby trapped in a “ship-in-a-bottle” fashion. Such structures were until recently very rare because there are so few existing MOMs with cages of the right size and shape. However, we have recently introduced a new approach to the synthesis of porph @ MOMs in which the MOM structure is templated by the porphyrin (i.e. the cage builds around the porphyrin).1-4 This approach has facilitated the generation of many new examples of porph @ MOMs.
How are Porph @ MOMs prepared?
In a typical reaction, porphyrins and other starting materials are reacted in a one step, one-pot synthesis. The key to success is to use a suitable porphyrin and to select metals and organic linkers that are known to be versatile in terms of the types of structures that they can form. Metals such as zinc, cadmium are particularly useful in this context.
Why are Porph @ MOMs of interest?
The structure of porph @ MOMs is such that although the porphyrin is trapped in its cage it is remains exposed to pores that connect to the outside world. This affords several consequences, all of them positive:
- The porphyrin moiety retains its properties and can engage in size-selective catalysis controlled by the pores.
- The porph @ MOM can be easily recycled by filtering the crystals of porph @ MOMs. This is a feature that cannot be readily accomplished when reactions are conducted in solution.
- Porph @ MOMs can be modified in several ways by taking advantage of the permanent porosity of the porph @ MOM and using a process called post-synthetic modification. For example, the metal in the MBB can be changed, the metal in the porphyrin can be exchanged, the walls of the MOM can be modified. This allows for fine-tuning of properties in a systematic manner. This can change the size and molecular recognition properties of the pores in the porph @ MOMs.
- Further, the MBBs used to prepare the MOM structure are inexpensive and the porphyrin can be relatively cheap because commercially available porphyrins can be used.